Dilution of sample →Culture and counting →Results and Reports
Dilution of sample
Solid and semisolid samples
Weigh 25g into a sterile homogenizing cup containing 225 mL phosphate buffer or normal saline and homogenize at 8000 r/min to 10000 r/min for 1min to 2min, or put it into a sterile homogenizing bag containing 225 mL diluant and beat it with a slapping homogenizer for 1min to 2min. Make 1:10 sample homogenate.
The liquid sample
Take 25mL sample with sterile pipette and place it in a sterile conical flask containing 225mL phosphate buffer or normal saline (with appropriate number of sterile glass beads preset in the flask), mix thoroughly, and make 1:10 sample homogenization solution.

After the completion of the previous step, 1mL of 1:10 sample homogenate was sucked with 1mL sterile pipette or micropipette, and slowly injected into the sterile test tube containing 9mL diluent along the tube wall (note that the pipette or suction tip should not touch the diluent level). The test tube was shaken or another sterile pipette was used to repeatedly blow and mix it evenly, and 1:100 sample homogenate was made.

The equipment used in pipetting should be free from microbial contamination. Use a high-pressure resistant pipette gun to check the sterility of the ear washing bulb and avoid touching the homogeneous bag or the inner wall of the test tube with the pipette gun...
After the homogenization of the sample is completed, a series of 10 times diluted sample homogenization can be prepared by the same operation. Use 1 ml sterile pipette or suction head for each incremental dilution.
According to the estimation of the contamination status of samples, 2 ~ 3 sample homogenates with appropriate dilution (liquid sample can include stock solution) were selected, and 1 mL sample homogenate was absorbed into sterile plates during 10-fold incremental dilution, and two plates were made for each dilution. At the same time, 1 mL blank diluent was absorbed and added to two sterile dishes as blank control.
After that, 15 mL ~ 20 mL plate counting AGAR medium cooled to 46℃ (which can be placed in a constant temperature water bath at 46℃ ±1 ℃ for heat preservation) was poured into the plate in time, and the plate was rotated to mix evenly.
Culture and counting
Once the sample has been diluted, culture and colony counting are performed
After AGAR solidified, the plate was turned over and cultured at 36℃±1℃ for 48h±2h. Aquatic products were cultured at 30℃±1℃ for 72h±3h.
If the sample may contain diffuse colonies growing on the surface of the AGAR medium, a thin layer of AGAR medium (about 4mL) can be covered on the surface of the solidified AGAR, and the plate can be turned after solidification to culture under the same conditions.
After the culture is completed, it is time to count the colonies, which can be observed by the naked eye or, if necessary, by using a magnifying glass or a colony counter, to record the dilution factor and the corresponding number of colonies. Colony count is expressed as colony forming units (CFU).
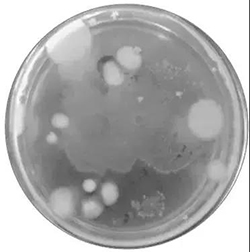
The total number of colonies was counted on plates with no spreading colonies between 30 CFU and 300 CFU. The number of colonies recorded on the plate was lower than 30 CFU, while that above 300 CFU could not be counted. The number of colonies per dilution should be the average of the two plates.
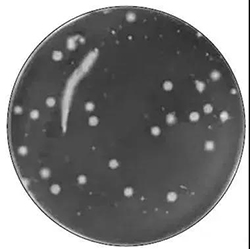
Large colonies
When one plate has large flake colony growth, it is not suitable to use, and the plate without flake colony growth should be used as the number of colonies in this dilution. If the number of flake colonies is less than half of the plate, and the other half of the plate is evenly distributed, half of the plate can be calculated and multiplied by 2 to represent the number of colonies in a plate.
Chain growth
When there is chain growth on the plate with no clear boundary between colonies, each single strand is counted as a colony.
Results and Reports
In order to generate results and reports, the total number of colonies needs to be calculated based on the count results and formulas. It is important to note that the counting principles are different for petri dishes with different numbers of colonies.
Colony spread is reported if all plates cannot be counted for spreading colonies. If there is colony growth on the blank control, the test result is invalid.
Weighing samples were reported in CFU/g, and volumetric samples were reported in CFU/mL.
Post time:2024-08-01




