Introduction to cell culture methods in channel microsope slides
This application note explains how to grow adherent cells in cell culture microchannels. Cell seeding, medium exchange and optical properties will be described. In addition, the main differences between the cell culture channel and the standard open well format are shown.
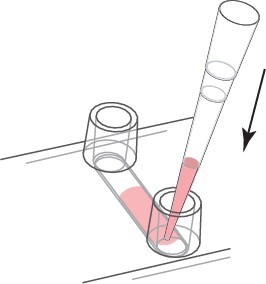
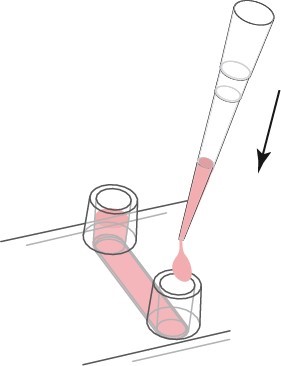
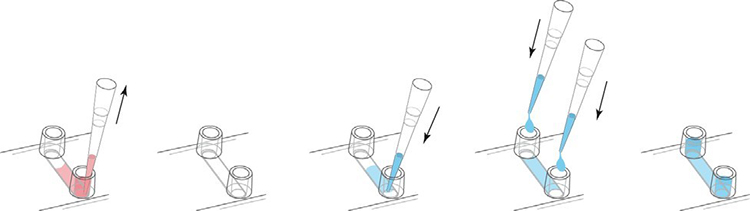
In order to show the cell seeding and μ-Slide treatment, the μ-Slide VI 0.4 was used for demonstration. Prepare the cell suspension as usual (eg 3×105 cells/ml) and add 30μl to the channel. Place the pipette tip on the entrance of the channel and point it towards the channel as shown. Quickly allocate and fill the entire channel.
After the cells are attached, fill each channel with 60 μl of cell-free medium as shown in the figure above. Take care to avoid air bubbles. If the filling step is performed after the cells have adhered, the cells will not be flushed out of the channel. If you want to fill the channel immediately after cell seeding, pipette carefully.
μ-Slide VI 0.4 is full of cells and culture medium during microscopic observation.
Fill the Luer reservoir of μ-Slide VI 0.4.
2. Medium exchange
a. Continuous medium exchange-recommended
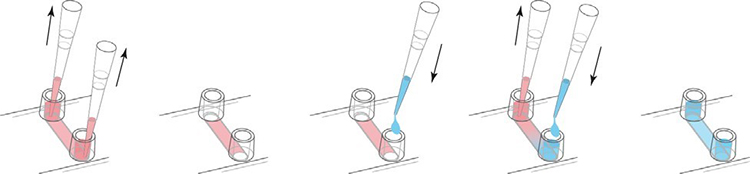
For continuous media replacement, first remove the old media from the storage. Then, add an appropriate amount of fresh medium to one reservoir while aspirating from the other reservoir. Use cell culture pipettes carefully. We recommend that the volume be at least 3 times the channel volume. Refill the reservoir.
The continuous exchange medium volume is 3 times the medium.
b. Complete culture medium exchange-only suitable for expensive liquids
If you only replace the liquid in the channel, please empty the channel first. Then, place the pipette tip on the channel entrance and carefully aspirate the liquid from the channel. Use a cell culture pipette to completely remove all liquid. In order to refill the channel, fresh medium of the channel capacity is directly injected into the channel. Avoid creating bubbles. Refill the reservoir holes on both sides.
Emptying the channel completely may cause bubbles to form after refilling.
The channels and storage holes are completely replaced with liquid.
3. Channel slides and porous slides
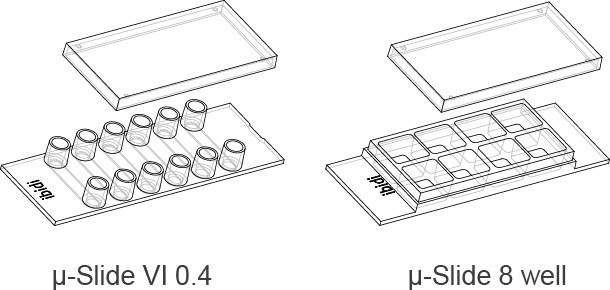
In the following section, we compare the characteristics of μ-Slide VI 0.4 (cell culture channel) with μ-Slide 8-well (open format).
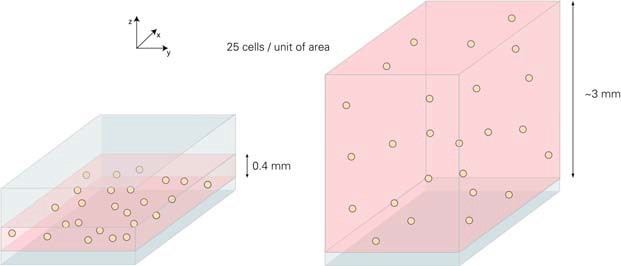
For seeded cells, the main difference is the height of the liquid inside the structure. The small area parts of the two systems are shown below. The distance between the bottom and top of the μ-Slide VI 0.4 channel is 400μm. The filled μ-Slide 8-well glass slide has no top structure. In the 8-well glass slide, the medium is about 0.3 millimeters, and the channel slide is 7.5 times thinner than the open format 8-well slide.
For cell seeding, different cell densities must be applied to obtain the same amount of cells on the surface. In this example, the goal is to seed 25 cells/unit area. Since the height of the liquid in the μ-Slide 8 well is 7.5 times, the applied cell concentration must be lower than the same factor.
Based on the different height of the liquid inside the μ-Slide VI 0.4 and μ-Slide 8 holes. In the above example, the same growth area and number of cells but different volumes result in different cell concentrations.
In order to obtain a considerable degree of fusion, we recommend using 3 … 7 x 105 cells/ml (μ-Slide VI 0.4) and 4 … 9 x 104 cells/ml (μ-Slide 8 wells). This is ~7.5 times the same between the two geometries. After cell adhesion, the number of cells per unit area is the same.
Due to different heights, different cell concentrations produce the same level of cell aggregation.
4. Advantages of channel μ-Slide
Compared with the standard aperture format, channel slides have strong advantages.
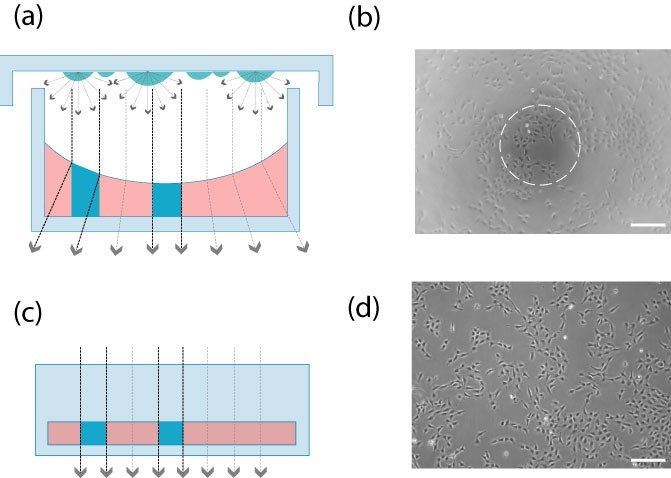
a. Better phase contrast microscopy imaging effect
The channel geometry is very convenient to use a phase contrast microscope, because there is no influence of the concave liquid surface, the entire growth area can be observed with the phase contrast technique.
Unlike open porous slides, channel slides will not interfere with the beam path of the phase contrast microscope, resulting in better phase contrast effects.
Open multi-well culture plate (picture a, picture b): the meniscus affects the phase difference effect. Only the center part can provide high-quality contrast.
Channel slides (Figure c, Figure d): In the channel geometry, the beam path is always aligned. The entire field of view can provide high-quality phase contrast microscope imaging.
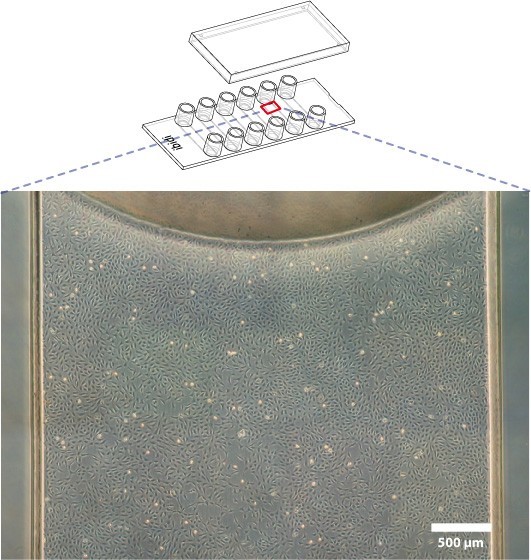
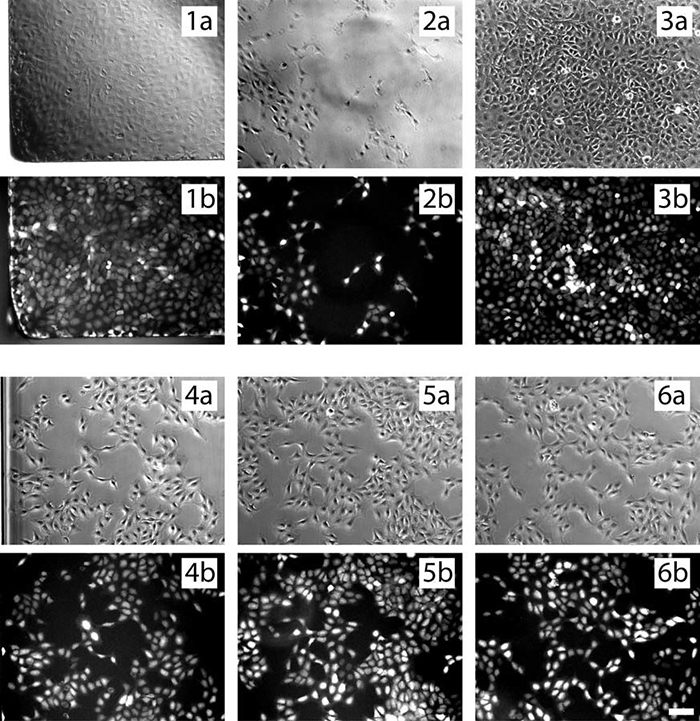
b. Cells are evenly distributed
In the channel, because the edge effect is small, the homogeneity of adherent cells is much better.
The following microscopic images show the uneven distribution of cells in the open wells (1-3) and the homogeneity of the cell culture channels (4-6) in the phase contrast (a) and fluorescence (b) modes.
Post time:2024-08-01